Vascular pathophysiology

C/ Irunlarrea 3
Navarrabiomed-Centro de Investigación Biomédica
Complejo Hospitalario de Navarra
31008 Pamplona, Navarra. España

C/ Irunlarrea 3
Navarrabiomed-Centro de Investigación Biomédica
Complejo Hospitalario de Navarra
31008 Pamplona, Navarra. España

Eva Jover García es licenciada en Ciencias Biológicas por la Universidad de Valencia y realizó su tesis doctoral en la Universidad de Murcia. Tras cinco años de investigación posdoctoral en la Universidad de Bristol (Reino Unido), se incorporó en el año 2020 a la Unidad de Cardiología Traslacional de Navarrabiomed, gracias a la obtención de un contrato posdoctoral Sara Borrell del Instituto de Salud Carlos III y, posteriormente, fue beneficiaria de la beca ‘Stop Fuga de Cerebros’ de Roche Farma.
Desde enero de 2025, es responsable de la línea de investigación en Arritmias de Navarrabiomed mediante un contrato Miguel Servet del Instituto de Salud Carlos III.
C/ Irunlarrea 3
Navarrabiomed-Centro de Investigación Biomédica
Complejo Hospitalario de Navarra
31008 Pamplona, Navarra. España
The Translational Medical Oncology Unit focuses on developing projects that facilitate the transition to precision medicine in the field of medical oncology in Navarra. To do this, we integrate translational research, understood as the link between the clinic and the laboratory, and basic research, understood as the study of the biology that characterises carcinogenesis and its progression. Our lines focus on the study of genetic alterations in both tumour biopsy and liquid biopsy, with the aim of identifying molecular biomarkers that facilitate the management of the disease. In addition, we study the immune component and its interaction with tumour cells, to characterise key cell populations in the development of new strategies in immunotherapy.
Lines of research:
Navarrabiomed-Centro de Investigación Biomédica
Hospital Universitario de Navarra, edificio de investigación.
C/ Irunlarrea 3. 31008 Pamplona, Navarra. España
A cross-sectional and multidisciplinary unit focused on clinical and translational research of inflammatory and immune-mediated diseases, including autoinflammatory and autoimmune diseases. It promotes its own studies and participates in both national and international multicentre projects, with an observational and interventional design. It has been a promoter of both funded and independent clinical trials.
Lines of research:
C/ Irunlarrea 3
Navarrabiomed-Centro de Investigación Biomédica
Complejo Hospitalario de Navarra
31008 Pamplona, Navarra. España

The Translational Cardiology Research Unit is made of scientists who do basic research at the Navarrabiomed biomedical research centre and clinical researchers from the Clinical Cardiac Area at the Navarra Hospital Complex. The team’s primary goal is to study novel therapeutic targets for different types of cardiovascular diseases such as heart failure, aortic stenosis, aortic insufficiency or mitral valve disease.
This Unit carries out research projects in collaboration with the CIC at Nancy, the INSERM U1138 research centre based in Paris, the INSERM UMR1048 from Toulouse (France), and the Complutense University of Madrid.
Lines of research:

The doctoral work, entitled "Role of sST2 in myocardial fibrosis in severe aortic stenosis”, has been developed at the University Hospital of Navarra and Navarrabiomed under the direction of Natalia López Andrés, Principal investigator of the Translational Cardiology Unit.
Aortic stenosis is the most common valvular heart disease in Europe and North America affecting 2-7%, depending on the region, in population over 65 years of age. To date, there is no medical treatment that can slow down or reverse the evolution of the disease, so aortic valve replacement (surgical or percutaneous) is the only treatment when symptoms or ventricular dysfunction appear.
This disease produces an abnormal progressive narrowing of the aortic valve that, as a result of pressure overload, causes hypertrophy of the left ventricle. In this process, myocardial fibrosis has an important pathophysiological role, as well as a prognostic role. Initially, myocardial fibrosis is part of a compensatory mechanism, but in advanced stages a focal replacement fibrosis appears, leading to ventricular dysfunction and heart failure. The pathophysiological mechanisms underlying these processes are not fully understood.
Focal replacement fibrosis can be detected and quantified by cardiac magnetic resonance imaging (MRI) with the delayed enhancement (DE) sequences. The presence of DE in patients with severe aortic stenosis has been shown to be an independent predictor of mortality and unfavourable clinical outcome in this group of patients. However, MRI is an expensive technique with limited availability, so it is not used in the follow-up of this group of patients in routine clinical practice.
The hypothesis of this thesis is that as the levels of soluble ST2 (sST2), a biomarker associated with the process of fibrosis and myocardial remodelling, are elevated in case of aortic stenosis, they may have a prognostic value. Specifically, this study addresses the role of sST2 in myocardial fibrosis in severe aortic stenosis.
Research development
The work is proposed from a translational point of view, and has a dual goal. First of all, to delve into the pathophysiological role of tSS2 in severe aortic stenosis. To this end, a proteomic study has been carried out to assess the proteins modulated by sST2 in human cardiac fibroblasts and the in vitro effects of sST2 on human cardiac fibroblasts have been investigated. The results have been validated in vitro in a rat model with pressure overload and in myocardial biopsies of patients with aortic stenosis that underwent surgery.
Likewise, it has been demonstrated that sST2 exerts a deleterious role in human cardiac fibroblasts, on the one hand, affecting the mitochondrial function of the cell and thus increasing oxidative stress and the synthesis of proinflammatory molecules and on the other hand, promoting differentiation to myofibroblasts and increasing the synthesis of profibrotic molecules. These findings were validated in the animal model and in myocardial biopsies of patients with aortic stenosis.
Secondly, from the clinical point of view, a cohort of patients with severe aortic stenosis with surgical indication was analysed to check if the blood levels of sST2 are associated with the DE evaluated by MRI in patients with severe aortic stenosis. Thus, it is observed that patients with severe aortic stenosis with cardiac MRI DE have significantly higher blood levels of sST2 than those without RT. Blood sST2 levels are positively correlated with DE mass and with VI mass in patients with severe aortic stenosis. High levels of sST2 make it possible to identify patients with severe aortic stenosis with DE, without having to perform cardiac MRI, in a simple way that can be applied in routine clinical practice.
Dissemination of results
The work carried out has led to several scientific publications: in 2019, in the journal Clinical Science, “Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: an in vitro and in vivo study in aortic stenosis”, and in 2020 in the journal Cells, “Soluble St2 Induces Cardiac Fibroblast Activation and Collagen Synthesis via Neuropilin-1”.
In addition, the results have been disclosed at several national and international congresses such as the SEC Congress in Bilbao (in 2015 and in 2017), at the 29th EACTS Annual Meeting in Amsterdam, in 2015 or at the Heart Failure Congress in Paris, in 2017.


Biochemist Jaime Ibarrola Ulzurrun (Pamplona, 1991) has shown for the first time that a hormone is involved in mitral valve prolapse, a heart valve disease that leads to heart failure, and that a series of drugs can have positive effects on this condition. ‘The drugs known as antimineralocorticoids or mineralocorticoid receptor antagonists (MCRAs) are a promising option to reduce mitral valve remodelling. The only existing solution to date was surgery,’ Ibarrola explains. This was the subject of his doctoral thesis at the Public University of Navarra (UPNA).
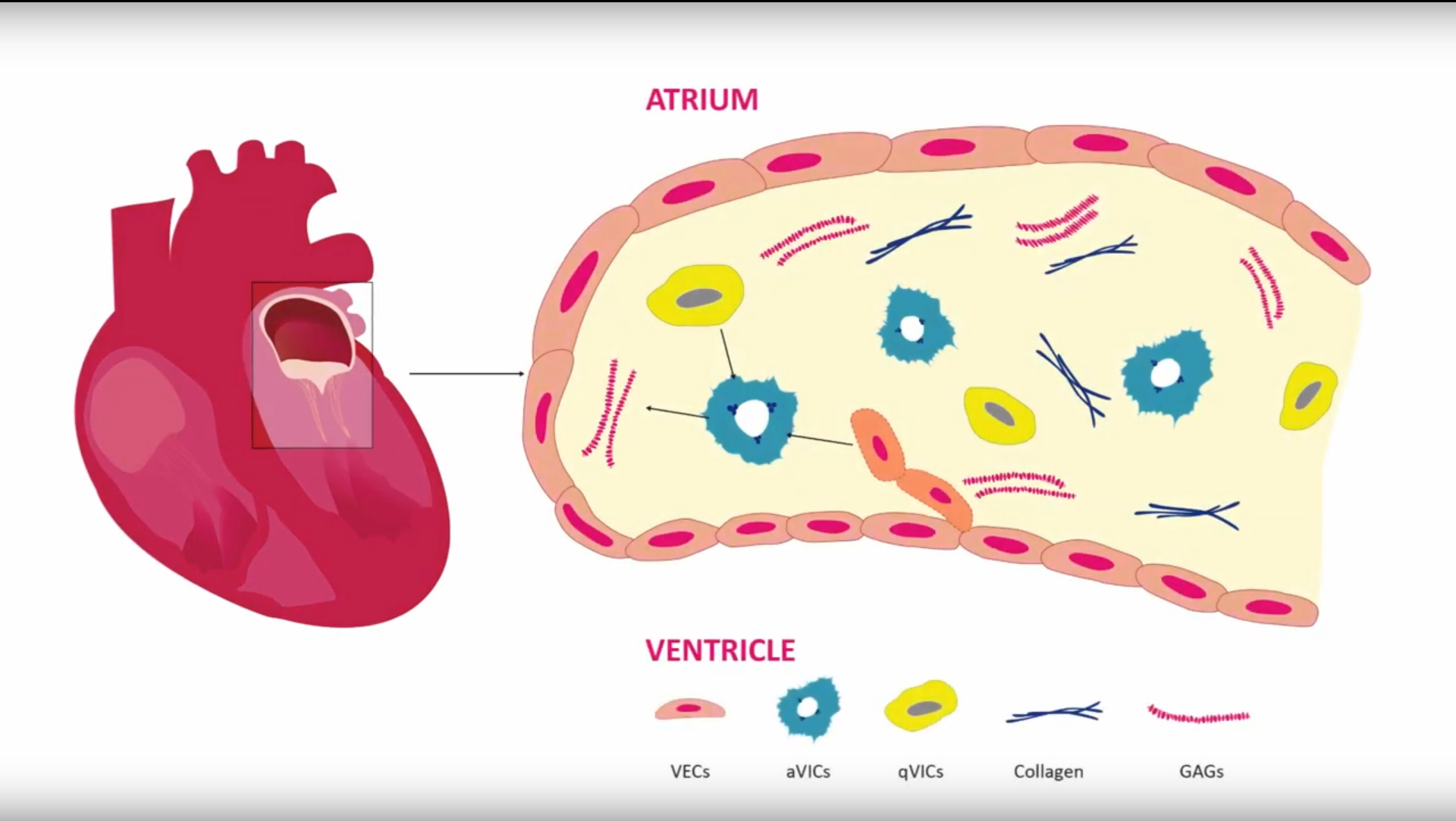
The mitral valve is the valve between the left atrium and the left ventricle of the heart. Mitral valve prolapse is a condition in which the two valve flaps of the mitral valve do not close smoothly or evenly, but instead bulge (prolapse) upward into the left atrium. Sometimes, the mitral valve does not close tightly, allowing blood to flow backward in the heart. This condition is known as mitral valve regurgitation or mitral insufficiency. Most people with mitral valve prolapse – one of the most common heart conditions, affecting 176 million people around the world – never have problems. They do not need treatment or lifestyle changes. Some, however, do need to be treated. ‘To date, no drugs have been developed for this condition, so the only viable solution is surgery,’ Ibarrola explains. His doctoral advisor was Natalia López Andrés, senior researcher at the Cardiovascular Translational Research Unit of Navarrabiomed, a joint centre of the Government of Navarra and the Public University of Navarra (UPNA).
New therapeutic targets
Ibarrola’s research responds to the need to study ‘new mechanisms and new therapeutic targets in order to find drug treatments for mitral valve prolapse.’ ‘Mineralocorticoids are a class of hormones produced by the human body. The primary mineralocorticoid is aldosterone. The aldosterone/mineralocorticoid receptor (Aldo/MR) pathway can cause cardiac fibrosis. In addition, a large number of studies have shown that the Aldo/MR pathway is involved in a number of heart conditions. MCRA drugs can block the effects of the Aldo/MR pathway. Moreover, significant clinical studies show that they can also improve cardiac function by reducing cardiac fibrosis,’ Ibarrola explains. He conducted his doctoral research project with financial aid from UPNA and a European programme.
Ibarrola worked on the hypothesis that the Aldo/MR pathway could play a role ‘in the development of mitral valve prolapse, modulating cell activation and cellular differentiation.’ ‘Furthermore, the Aldo/MR pathway could become a new therapeutic target in this disease, and blocking this pathway with MCRA drugs could prevent the alterations associated with mitral valve prolapse. For the first time, we were able to show that the Aldo/MR pathway is involved in the development of mitral valve prolapse and that the drugs could have a positive effect on this condition,’ Ibarrola concludes. His doctoral thesis got an A-grade cum laude.
Ibarrola’s résumé
Jaime Ibarrola holds a degree in Biochemistry and a master’s degree in Biomedical Research from the University of Navarra. At present, he is a postdoctoral researcher at the Molecular Cardiology Research Institute (MCRI) at Tufts University (Massachusetts, USA).
As a doctoral student, Ibarrola was twice a visiting scholar at the Cordeliers Research Centre, Sorbonne University, in Paris. He shared his results in eight international conferences in Germany, Spain, France and Ireland. Ibarrola is the author of about a dozen papers published in international scientific journals.

Navarrabiomed - Centro de investigación biomédica
Complejo Hospitalario de Navarra, edificio de investigación.
Calle Irunlarrea, 3. 31008 Pamplona, Navarra, España.
The Microbial Pathogenesis Research Unit seeks to understand at the molecular level how pathogenic bacteria grow when adhering to the surface of medical devices and tissues, leading to infections that are resistant to antibiotics and therefore tend to become chronic. In order to understand this form of bacterial growth, known as biofilm, genetic engineering strategies are used, along with omics approaches, synthetic biology and animal models.
The ultimate goal is to identify the critical elements in biofilm formation in order to prevent biofilm from forming, eliminate already formed biofilm, improve existing treatments and favouring the formation of non-pathogenic bacteria biofilm for therapeutic purposes.
Lines of research:
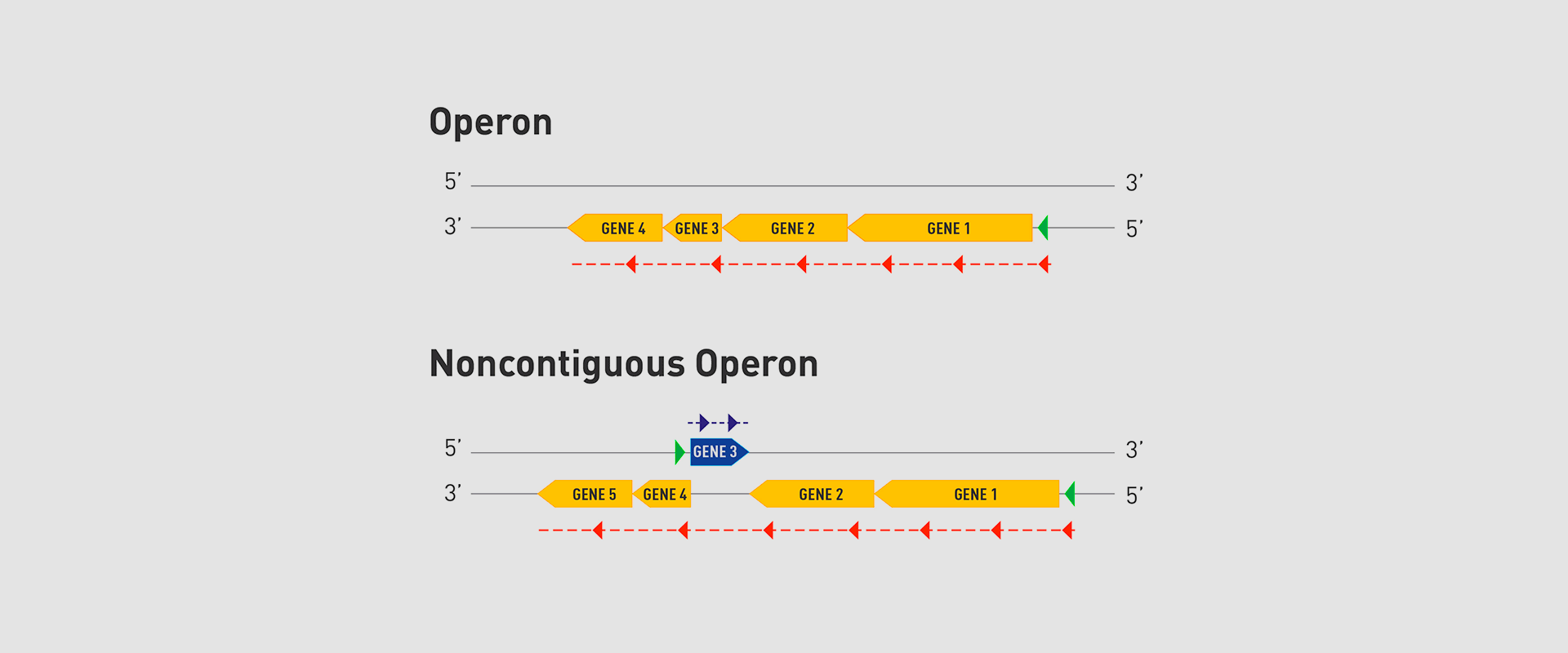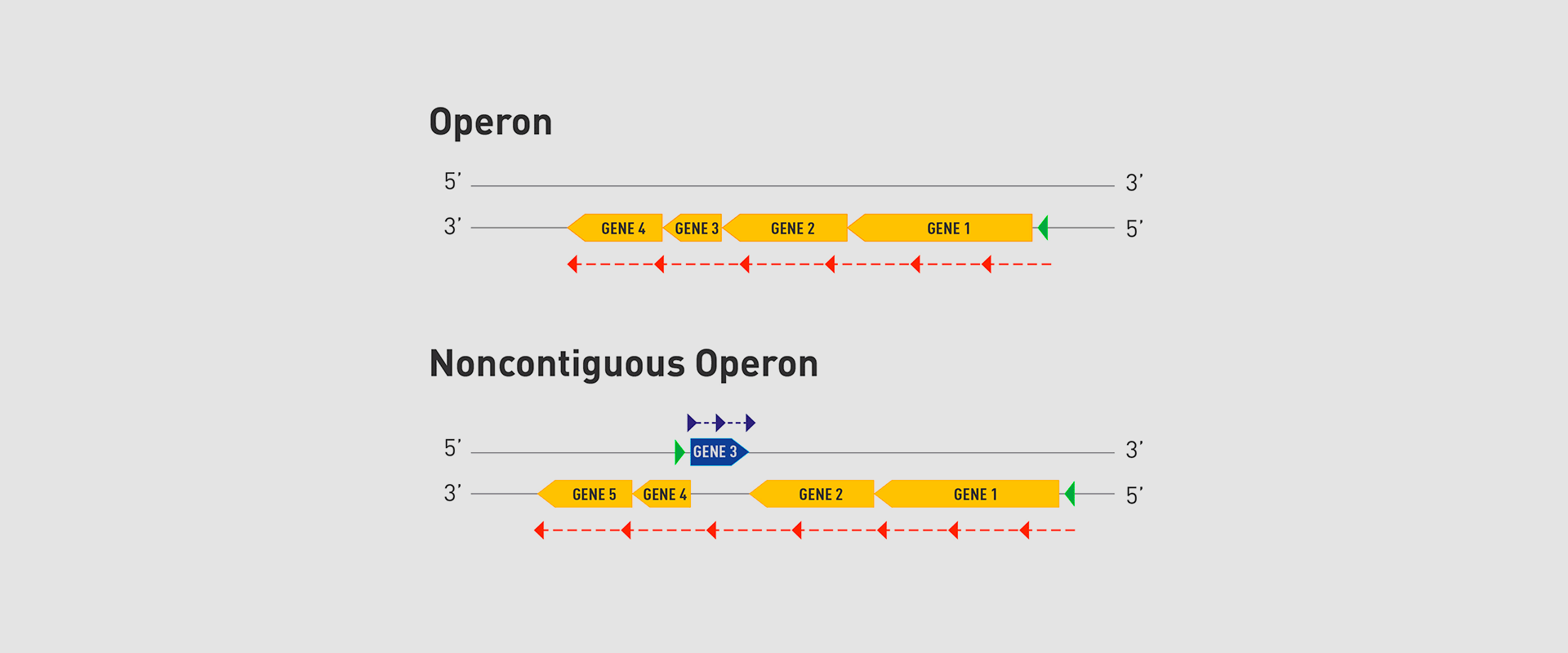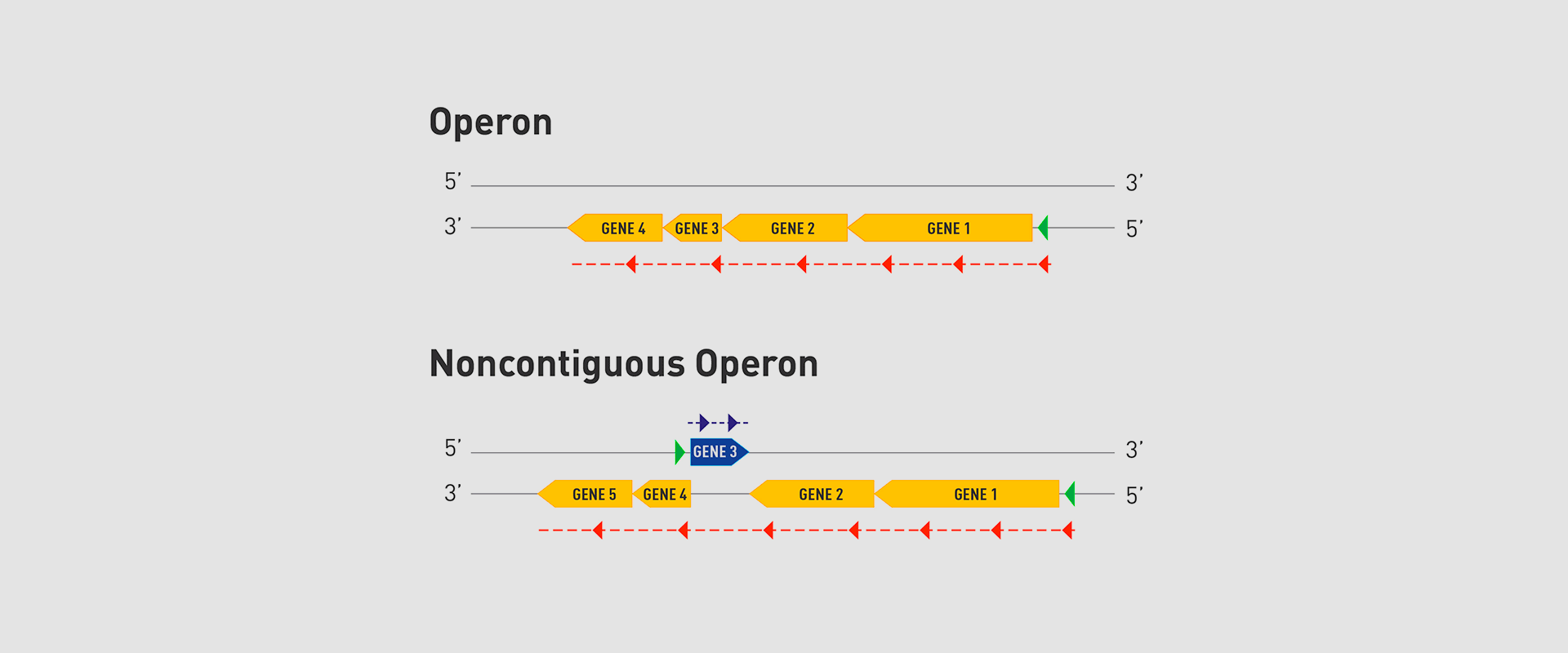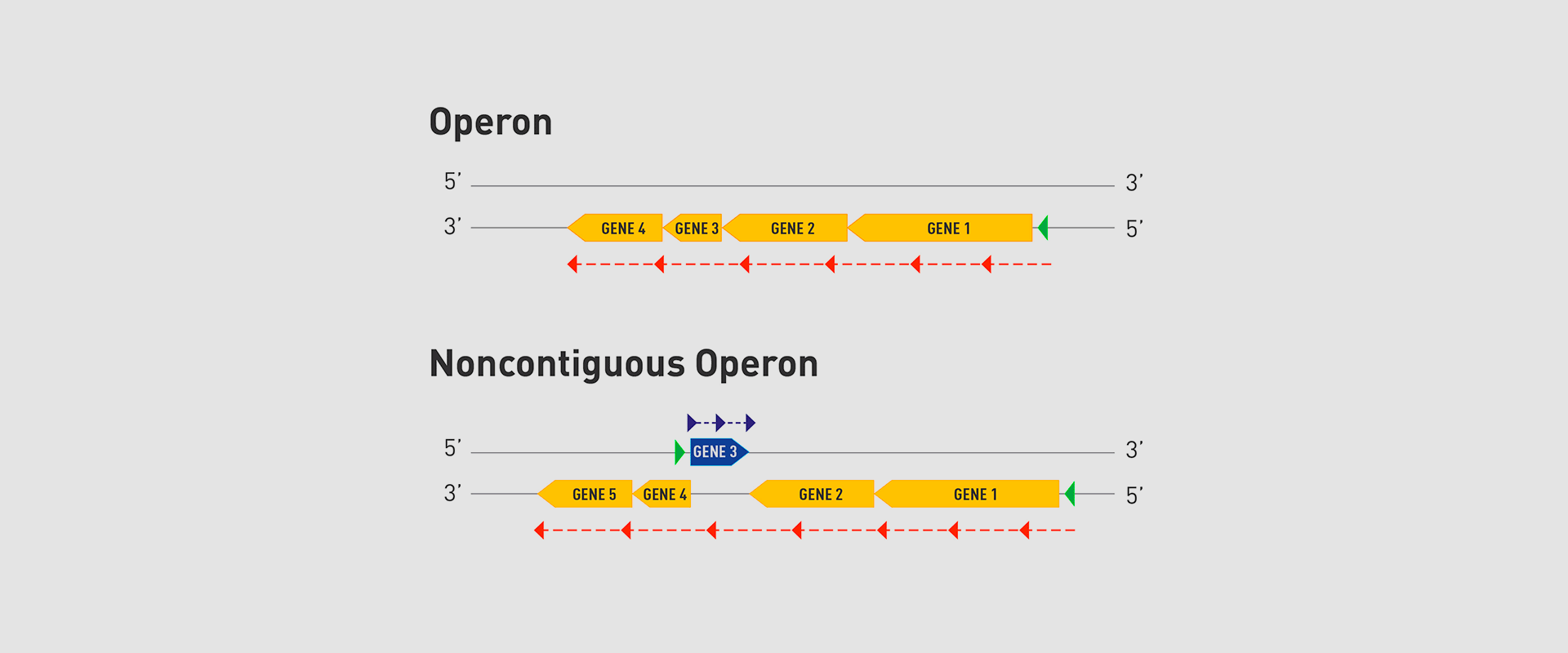
The Microbial Pathogenesis Unit of Navarrabiomed-Public University of Navarra (UPNA) has found a new genetic organisation in bacteria that helps better understand bacterial biology. The study of this genetic architecture was published in Proceedings of the National Academy of Sciences of the United States of America (PNAS).
Research background
In 1961, François Jacob and Jacques Monod discovered that bacteria group the genes that encode the proteins for a certain metabolic pathway in a single transcription unit (which they called ‘operon’). They won the Nobel Prize in Physiology or Medicine in 1965 for their discovery.
The bacteria they chose for their study was Escherichia coli, which normally lives in the intestines of healthy people; specifically, they studied the set of genes E. coli bacteria need to transport lactose (milk sugar) and break it down. E. coli only produces the three proteins it needs to digest lactose when the sugar is available. To simplify transcription regulation, the three genes involved are adjacent in the genome and under a single regulation system. Similar transcriptional regulation systems are found in other metabolic pathways in all bacteria.
Research at Navarrabiomed
In 2018, the team of researchers at Navarrabiomed coordinated by Iñigo Lasa Uzcudun, Head of the Microbial Pathogenesis Unit and Director of the biomedical research centre, described a new way genes are organised in bacteria. This regulation system has a higher level of regulation in operon structure, which the authors of the study named ‘non-contiguous operon’.
The bacterial model analysed has a group of four genes that are transcribed as a transcription unit despite the existence of a separate gene between the second and third genes that is transcribed in the opposite direction.
This transcriptional architecture results in an antisense transcript that acts as a mutual regulation system for the expression of the genes in the operon and the gene that produces this antisense transcript. Therefore, the concept of non-contiguous operon includes not only the genes transcribed from the same transcription unit but also overlapping genes whose expression is coordinated with that of the genes in the operon.
This finding deepens the understanding of bacterial biology and may trigger novel developments in the fields of synthetic biology and bacterial biotechnology.
The study was carried out as part of the scientific activity done at the Navarra Medical Research Institute (IdiSNA).

Un equipo científico del centro de investigación biomédica Navarrabiomed -centro mixto del Gobierno de Navarra y la Universidad Pública de Navarra (UPNA)- ha conseguido caracterizar el sistema sensorial que las bacterias utilizan entre otras cosas para multiplicarse en el cuerpo humano y causar infección.
El avance, que ha sido publicado por la revista científica Nature Communications y cuenta con financiación del Ministerio de Economía, Industria y Competitividad, permite comprender mejor cómo las bacterias se adaptan a las diferentes condiciones ambientales y posibilitará el desarrollo de antibióticos más específicos y eficaces.
El estudio ha contado con el liderazgo del doctor Iñigo Lasa, director de Navarrabiomed e investigador responsable del Grupo de Patogénesis Microbiana del centro. Asimismo, han colaborado investigadores del Instituto de Agrobiotecnología (UPNA-CSIC-Gobierno de Navarra), del Instituto de Biomedicina de Valencia (CSIC) y del Institute of Infection, Immunity and Inflammation, University of Glasgow.
Bacterias superresistentes
Actualmente, la aparición de bacterias farmacorresistentes, que no responden a tratamientos con antibióticos, constituye uno de los problemas sanitarios a escala mundial priorizados por la Organización Mundial de la Salud (OMS).
Las bacterias detectan, responden y se adaptan a los cambios en su entorno utilizando unos elementos sensoriales denominados sistemas de dos componentes. Este tipo de sistemas sensoriales están presentes en bacterias, hongos y plantas, pero no se encuentran en células animales. En el caso de las bacterias, regulan procesos celulares tan importantes como la virulencia o su propio crecimiento, lo que los convierte en dianas para el diseño de nuevas terapias antimicrobianas.
El objetivo del trabajo ha consistido en eliminar todos los sistemas de dos componentes, es decir el sistema sensorial completo, en Staphylococcus aureus, uno de los principales patógenos humanos según la OMS y, posteriormente, en la generación de una colección de bacterias cada una de las cuales contiene un único sistema de dos-componentes. Esta estrategia ha permitido simplificar una compleja red sensorial en cada uno de sus elementos para comprender cuál es la función individual de cada uno de los sistemas y la relación existente entre ellos.
Aplicación clínica de la investigación
En relación a la aplicación clínica, Iñigo Lasa apunta al desarrollo de nuevos antibióticos más específicos. “El hecho de que los sistemas de dos componentes estén presentes en todas las bacterias patógenas y no en las células de nuestro organismo nos puede permitir desarrollar fármacos que bloqueen estos sistemas, evitando así el desarrollo de la bacteria durante la infección, sin causar ningún efecto secundario sobre nuestras células”.
En este sentido, las bacterias generadas en este estudio han sido patentadas y actualmente el equipo analiza diversos compuestos marinos que puedan incorporarse en el tratamiento y control de infecciones en la práctica clínica.
La investigación forma parte de la actividad científica del Instituto de Investigación Sanitaria de Navarra (IdiSNA), agrupación público-privada para el fomento de la investigación biomédica en la Comunidad Foral y de la que son miembros Navarrabiomed y la UPNA.

Navarrabiomed - Centro de investigación biomédica
Complejo Hospitalario de Navarra, edificio de investigación.
Calle Irunlarrea, 3. 31008 Pamplona, Navarra, España.

The Genomic Medicine Unit implements whole genome sequencing (WGS) methods for analysing entire genomes in the public health system. WGS is a powerful clinical, research and development tool in the field of precision medicine in Navarra. Emerging from research projects funded by the Department for Industry of the Government of Navarra, the Genomic Medicine Unit brings together its own staff (director, genetic advisor, geneticist, lab technician), clinical experts from the Navarra Hospital Complex (coordinators from twenty medical specialties), and staff from other Units (Bioinformatics) and Platforms, as well as advisors. At present, this Unit is carrying out four major research projects. Its core helps support future developments in this area.
Lines of research:
High-cholesterol levels, or hypercholesterolemia, affects nearly 1500-2500 people in Navarra, and less than 20 per cent of them are not aware that they suffer from this condition. Hypercholesterolemia is considered to cause 22 per cent of coronary events, most of which could be prevented with early diagnosis and treatment. The biomedical research centre Navarrabiomed has launched NAGENCOL, a project to address this issue using whole-genome sequencing as a diagnostic tool to offer personalised treatment to patients who suffer from hypercholesterolemia. The project is framed within NAGEN, a global strategy aimed at applying genomic medicine in the Navarra Health System-Osasunbidea (SNS-O).
Currently, hypercholesterolemia poses a real challenge to the public health system, because the life expectancy of untreated patients can decrease by 25 years, and 50 per cent of them are more likely to have a heart attack before the age of 55. NAGENCOL addresses this problem, offering a new model that uses genomic information, together with other clinical and demographic data, to bring precision medicine to individual patients.
This ambitious public health project has a budget of 2 million Euro, contributed by the Department of Economic Development at the Directorate-General for Industry, Energy and Innovation of the Government of Navarra, within the framework of the Genomics and Advanced Medicine project (GEMA) and the Intelligent Specialisation Strategy S3.
The NAGENCOL activities are managed by five strategic partners specialising in clinical practice, scientific and technical services, and research. They include the Navarra Hospital Complex (CHN), Nasertic, Tracasa Instrumental SL and Navarrabiomed as the leader of the study. The project is headed by Dr Ander Ernaga and Dr Juan Pablo Martínez from the Endocrinology Service at CHN.
NAGEN Strategy
Since 2016, Navarrabiomed has been leading the Genomic Medicine Strategy (NAGEN) of the Navarra Health System-Osasunbidea. With the support of the Government of Navarra, Navarrabiomed has since coordinated two strategic projects: NAGEN 1000 (best precision medicine project award winner in 2018) and Pharmanagen.
The two initiatives, along with NAGENCOL, are being used to set up, in the SNS-O patient care units, the infrastructure required for using genomic data as a powerful diagnostic tool and to determine the best personalised treatment for each patient.


Navarrabiomed has led the NAGEN: Navarra Genome 1000 project since 2016. The project focuses on whole genome sequencing for a new approach to rare genetic disorders in the Navarra Health System-Osasunbidea (SNS-O). So far, thanks to collaboration with doctors from 18 medical specialties at the Navarra Hospital Complex (CHN), the study includes data about 400 patients and their relatives, precise diagnoses for 25 per cent patients and identification of possible causes for another 25 per cent.
The patients that are part of the study had not been accurately diagnosed, despite having been treated by several specialists and having taken a large number of traditional tests. Angel Alonso, the coordinator of the project, highlighted the project’s relevance to healthcare services: ‘Making genomic analysis available to the public health system is revolutionary. It means a significant change in the clinical approach to patients with rare genetic disorders.’
Impact on the patient and their family
On the occasion of Rare Disease Day on the last day of February, it is worth mentioning that about 6 per cent of the global population are individuals with rare diseases. In Navarra, their number amounts to 38,000. At present, there are 7000 types of rare diseases, most of them of genetic origin.
In many cases, genetic testing enables the patient and their family to get a deeper knowledge of their condition and its progression, to understand how a genetic disease is inherited and to learn about the risks for other family members. The emotional significance of finding answers to the questions posed by the symptoms – which sometimes have remained unanswered for too long – means putting an end to uncertainty and isolation for most patients with rare diseases.
NAGEN 1000: a pioneering project in Spain
NAGEN 1000 is a ground-breaking project at the national level, placing Navarra at the forefront of genomic analysis and technology. The project was introduced last year at the Senate Presentation of Genomic Studies, whose conclusions were approved in 2019, thus green-lighting the development of a national strategy for personalised medicine.
Currently, the project’s methods, procedures and infrastructure are being transferred to daily clinical practice in SNS-O, to the benefit of the people of Navarra.
NAGEN 1000 is financed by the Economic Development Department at the Directorate-General for Industry, Energy and Innovation within the framework of the Intelligent Specialisation Strategy S3. It is being developed by a consortium made of CHN, Nasertic (a company run by the Government of Navarra), Avantia and Navarrabiomed, leader and coordinator of the project, with the support of the Directorate-General for Information Technology, Telecommunications and Public Information (DIGITIP), and the cooperation of the Centro Nacional de Análisis Genómico (CNAG-CRG) and the Clinical Bioinformatics Research Area into Rare Diseases (CIBERER) of Instituto de Salud Carlos III (ISCIII).

Navarrabiomed - Centro de investigación biomédica
Complejo Hospitalario de Navarra, edificio de investigación.
Calle Irunlarrea, 3. 31008 Pamplona, Navarra, España.
The two main causes of death in cancer patients are metastasis and resistance to therapy. The Signalling in Cancer Unit seeks to understand both the molecular mechanisms involved in the formation of secondary tumours (metastasis) and the strategies used by cancer cells to resist chemotherapy drugs and targeted therapies.
The ultimate goal of the Unit is to transfer results to clinical practice.
A study led by Navarrabiomed proposes a therapeutic alternative to treat melanoma.
The prestigious journal Nature Metabolism has published the results of a study in mice that determined that ranolazine, a drug that is currently administered to patients to treat heart conditions, delays the appearance of resistance to melanoma treatments, by blocking fatty acids metabolism. This research has been led by Navarrabiomed, together with the Institute for Neurosciences (CSIC-UMH) and IRB Barcelona. Melanoma is the most aggressive type of skin cancer and, although it only accounts for 10% of skin cancer cases, it is responsible for 90% of deaths associated with skin tumours. Thanks to the development of targeted therapies and immunotherapies, the clinical management of patients affected with this type of cancer has improved, however, these therapies still have limitations because 50% of patients do not respond adequately and even develop resistance.
The evidence suggests that this resistance could be linked to metabolic reprogramming in cancer cells that is associated to changes in the way in which cells process and use nutrients. This research demonstrates that fatty acid metabolism plays an important role in the development of resistance to melanoma treatments.
Researchers have confirmed that increased fatty acid oxidation occurs during long-term treatment with BRAF inhibitors, one of the key genes in tumour progression, contributing to therapy resistance.
Ranolazine increases the efficacy of targeted therapy against melanoma because it can target fatty acid oxidation. In addition, the application of this drug promotes that melanoma cells become more visible to the immune system,improving the response to immunotherapies and increasing the ability of lymphocytes to control tumour growth.
A multicenter investigation
The Navarrabiomed Cancer Signaling Unit, directed by Imanol Arozarena Martinicorena, has coordinated the course of the research and has been in charge of carrying out the experiments related to resistance to targeted therapies and the study of how ranolazine affects the immunogenicity of melanoma cells.
Researchers at the laboratory led by Berta Sánchez-Laorden, belonging to the Cell Plasticity in Development and Disease group at the Institute for Neurosciences, have developed immunotherapy experiments in mice and have carried out the study of immune cells in the tumour microenvironment.
In addition, the IRB Barcelona Stem Cells and Cancer research group, led by Salvador Aznar-Benitah, has carried out individual cell RNA sequencing analyses, which have made it possible to find out in detail the effect of the drug on the state metabolism of tumour cells.
Funding
This study, which has been made possible thanks to funding granted by the Ministry of Science and Innovation, the Carlos III Health Institute, the Government of Navarra, the Spanish Multidisciplinary Melanoma Group (GEM), and the Melanoma Research Alliance, is a clear example of how basic research can contribute a lot to the repositioning of drugs, which makes it possible to significantly shorten the deadlines for providing answers to patients suffering from diseases as prevalent as cancer.



On October 27th, 2019 Breast Cancer Association of Navarre SARAY collected 75,000 euros in the solidarity race celebrated in Pamplona. 49,970 euros will fund two studies developed by Navarrabiomed on metastatic breast cancer.
One of the projects is focused on liquid biopsy in this type of cancer, and it is led by Natalia Ramírez Huerto, the head of the Hematological Oncology Unit of Navarrabiomed. SARAY´s contribution for the second year of the project development raises to 24,970 euros. Other study lead by Cancer Signaling Unit head Imanol Arozarena Martinicorena will receive 22,000 euros. His project aims to develop new adjuvant therapies to treat brain and bone metastases from these tumors. The additional funds will be donated to the Chronos Hope Project from Solti Foundation.
On the 25th of June SARAY decided to allocate the incomes in a face-to-face and online assembly among 725 associates (event delayed three months later due to the COVID-19 crisis). During the last years, the organization shows a strong commitment with biomedical research performed in Navarre. Therefore, it is relevant to highlight that since 2015 SARAY has increased by 65% its annual investment in oncology research.

Imanol Arozarena, senior researcher from the Cancer Signalling Unit at the Navarrabiomed biomedical research centre and a member of Instituto de Investigación Sanitaria de Navarra (IdiSNA), has recently published a melanoma review with University of Manchester Professor Claudia Wellbrock.
The two scientists were asked by the renowned scientific journal Nature Reviews: Cancer to write an article on the latest findings in melanoma cell distinct phenotypes and their relevance for melanoma development and response to therapy.
As many as 4000 new melanoma cases are diagnosed in Spain every year. Malignant melanoma is a serious type of skin cancer that is usually metastatic. It is notorious for its intra- and inter-tumour heterogeneity.
Such variability results from the ability of melanoma cells (melanocytes) to adjust to changing environmental conditions by reprogramming their genetic expression. Thus, melanocytes develop resistance to both targeted therapy and immunotherapy, and their plasticity plays a role in metastasis development.
In their article, the authors reveal the importance of combining recent developments in genomic technologies and the availability of large gene expression datasets for a precise definition of the gene signatures that characterise changes in each patient’s tumours and the prognostic relevance for tumour development and response to therapy.
According to Arozarena, ‘By understanding the molecular mechanisms that adapt melanoma cells to anti-tumour drugs, we will be able to prevent both therapy resistance and the progression of metastatic melanoma.


Navarrabiomed - Centro de investigación biomédica
Complejo Hospitalario de Navarra, edificio de investigación.
Calle Irunlarrea, 3. 31008 Pamplona, Navarra, España.
The Oncoimmunology Research Unit develops gene vaccines for cancer treatment. It analyses their effects in inhibitory cells of the immune system, such as myeloid-derived suppressor cells, which appear in cancer patients and favour tumour progression and metastasis. The team have shown that treatment with a lentiviral vector that expresses an immunostimulating cytokine, a PD-L1 silencing microRNA and a tumour antigen inhibits the function of myeloid-derived suppressor cells and is effective against melanoma.

Navarrabiomed carried out a study in which the status of the immune system was evaluated in lung cancer patients before and during immunotherapy. The study showed that the quantities and diversity of immune cells (myeloid cells) in blood from patients who responded to immunotherapies was comparable to that of healthy individuals. Moreover, the researchers found that elevated concentrations of fractalkine were found in these patients. Fractalkine is a protein required for maintaining an active, functional immune system. These findings could lead to the development of new treatments and more efficacious immunotherapies by using this protein in conjunction with current therapies.
The results were published in the journal EMBO Reports. The project was coordinated by Dr. Ana Bocanegra and Dr. Grazyna Kochan, researchers at the Onco-Immunology Unit of Navarrabiomed headed by Dr. David Escors. The study was carried out in close collaboration with the department of Medical Oncology at Hospital Universitario de Navarra (HUN) led by Dr. Ruth Vera, and it was funded by grants from the Spanish Association Against Cancer, Carlos III Health Institute-ERDF and the Government of Navarra’s Ministry of Economic and Business Development.
Research development
The study identified fractalkine as a biomarker of response by associating elevated concentrations of the protein with a better response to immune checkpoint blockade therapies. This protein was also presented as a new therapeutic agent capable of increasing the efficacy of PD-1 immune checkpoint blockade therapies in animal models of lung cancer that were previously resistant to this therapy.
The authors reported that therapies that are more efficacious could be developed from these results in the medium/long term by using fractalkine to stimulate immunoreactivity and thus improve the response to immunotherapy.
“These results confirm the need for a functional immune system prior to the administration of immunotherapies and, most importantly, they open up a line of research in which the anti-tumor action of fractalkine can be enhanced. In the long term, fractalkine treatment in combination with immunotherapies could be assessed in clinical trials,” said Navarrabiomed researcher Grazyna Kochan.
Collaborative study
The research team from Navarrabiomed and HUN collaborated with multidisciplinary groups from Navarra, La Rioja and Madrid coordinated by professionals with a proven track record in cancer research and clinical care, including: Dr. Rubén Pío, Dr. Luis Montuenga and Dr. Juan José Lasarte from Cima Universidad de Navarra, Dr. Alejandra Roncero from Hospital Universitario San Pedro (Logroño, La Rioja), Dr. Carolina Gotera from Hospital Universitario Fundación Jiménez Díaz (Madrid), Dr. Alfonso Ventura from Centro de Salud Salazar-Ezcároz (Navarra) and Dr. José Pichel from Centro de Investigación Biomédica de La Rioja (CIBIR, Logroño). Patients and their family members at the HUN and residents in Centro de Salud Salazar-Ezcároz (Navarra) also participated in the study.
Caption > From left to right: Luis Montuenga (Cima), David Escors and Grazyna Kochan (Navarrabiomed), Ruth Vera (HUN) y Rubén Pío (Cima). Absent in the photo: Ana Bocanegra (Navarrabiomed).


The Oncoimmunology Unit of Navarrabiomed, headed by Drs. Grazyna Kochan and David Escors in collaboration with the Oncobiona Unit, headed by Drs Ruth Vera and María Alsina, have characterized the memory T-cell responses against SARS in solid-tumour patients with previous SARS-CoV-2 infection followed by mRNA vaccination.
The study demonstrates that patients with solid tumours vaccinated with Bnt162b2 exhibit proficient antibody, T-cell and myeloid responses against the S1 protein of SARS-CoV-2 virus. Furthermore, patients with previous COVID-19 generate a potent memory T-cell response against S1 and M viral proteins. This indicates that the incorporation of the M protein in vaccine formulations could increase the efficacy of vaccines in cancer patients.
In addition, vaccination followed by a previous infection was also reported to markedly increase the immune response to the S1 protein. The study also highlight the exacerbated Th17 response after infection and vaccination in solid tumour patients, who already have baseline inflammation due to the disease. This suggests the requirement of further research in novel mRNA vaccine adjuvants to avoid this inflammatory response.
These results are part of the thesis by Miriam Echaide, PhD student of the Oncoimmunology Unit, and are included in the scientific production of the Navarra Health Research Institute (IdiSNA), a public-private group for the promotion of biomedical research in Navarra, of which Navarrabiomed is a member.
The research is funded by the European Union's Horizon 2020 Science and Innovation programme. The Oncoimmunology group has the additional support of other institutions such as the Spanish Association Against Cancer (AECC), the Carlos III Health Institute, the Department of Health and the Department of University, Innovation and Digital Transformation of the Government of Navarra and the Ministry of Science and Innovation.

David Escors Murugarren, the principal investigator and head of the Navarrabiomed Oncoimmunology Research Unit and an expert on coronavirus, was the winner at the annual SER Radio Network Awards in Navarre of the innovation award, which is sponsored by the company Viscofan. The jury applauded his research work on SARS-CoV-2, given that he leads a project whose mission is to create a platform for speeding up vaccine production in cases of pandemics like the current COVID-19 pandemic.
In his acceptance speech, Escors highlighted the value of the work done at research centers on a daily basis. “Our lab works every day in the fight against cancer and also against autoimmune diseases, which should not be overlooked, even though we’re now in an emergency situation,” he said. He expressed his gratitude, but also his surprise at winning the award and said, “There are many other scientists working every day, just like us. It’s our work, our passion, and it’s what we studied for and trained to do.” He ended his speech by thanking his family and friends for their support throughout his professional career.
The ceremony of the fifth edition of the SER Radio Network Awards in Navarre was held at the Baluarte Auditorium and hosted by journalist Joaquim Torrents. This year, COVID-19 and its consequences were particularly relevant and the jury naturally took them into account when deciding on the award winners. In total, 10 people or groups were recognized for their work in different facets of Navarre society.


The Spanish Ministry of Science and Innovation, through the Carlos III Health Institute (ISCIII), has awarded €232,000 to develop two public research projects within the context of the Navarre Health Research Institute (IdiSNA). David Escors Murugarren, a researcher at Navarrabiomed, and Jesús Castilla Catalán, a researcher at the Institute of Public and Occupational Health of Navarre (ISPLN), have received 100% of the amounts they applied for from the COVID-19 Fund, a mechanism approved by Royal Decree-Law 8 of 17 March 2020 on urgent extraordinary measures for dealing with the economic and social impact of COVID-19.
David Escors, the principal investigator at the Navarrabiomed Oncoimmunology Research Unit, began his scientific career working on coronaviruses at the Spanish National Biotechnology Center (CNB-CSIC), along with researcher Luis Enjuanes. He then continued his work at University College London (UCL) by applying lentiviral vectors and gene therapy in immunotherapy. He is a coronavirus specialist and the positive evaluation received from the ISCIII will enable him to obtain the €115,000 he applied for to develop the project “Platforms for developing biosafe SARS-CoV-2 vaccines.”
The aim of the initiative is to develop a platform for engineering biosafe vaccines for the virus that causes COVID-19 disease. The focus will be on the expression of viral proteins that may activate immunity. This line of research was started up specifically for COVID-19, given the current health emergency, but is based on the European ISOLDA Project - Horizon 2020 for generating more effective and safer virus vaccines (yellow fever, influenza and coronavirus) for adults over 65. Navarrabiomed has worked on this project since 2019 in coordination with professionals from the Spanish National Research Council (CSIC) and with Dutch, German and Italian collaborators.
Institute of Public and Occupational Health of Navarre
At the Institute of Public and Occupational Health of Navarre’s Group of Infectious Diseases and Vaccines, Jesús Castilla will lead the study “Infection, Hospitalization, ICU Admissions and Deaths Caused by SARS-CoV-2 in a Population Cohort.” To carry out the study, he will also receive the total amount applied for from the ISCIII: €117,000.
His proposal focuses on estimating the effect of sociodemographic characteristics, chronic diseases and other conditioning health factors on the risk of infection, hospitalization and severe forms of COVID-19. This will involve calculating the incidence of suspected cases, infections confirmed using PCR, hospitalizations, ICU admissions, assisted ventilation and mortality. The mortality rate will also be calculated in confirmed cases and hospitalizations. Antibody seroprevalence will also be evaluated in a sample of patients from the sentinel physician network and/or donors.
This is the second SARS-CoV-2 initiative for Jesús Castilla, given the ISPLN’s participation in the European project I-MOVE-COVID-19, with the involvement of 11 countries and 20 organizations. It is one of the European projects funded through the fast-track call of Horizon 2020, the European Union’s research and innovation program to promote research of different aspects of the SARS-CoV-2 virus.
Both research projects financed by the ISCIII will form part of the scientific activity of the IdiSNA, a public-private partnership for promoting biomedical research in Navarre. Both the ISPLN and Navarrabiomed are partnership members.

El Dr. Hugo Arasanz, oncólogo del Complejo Hospitalario de Navarra e investigador de la Unidad de Inmunomodulación de Navarrabiomed - IdiSNA, recibió ayer en Madrid la Ayuda Clínico Junior de la Asociación Española Contra el Cáncer (AECC). La financiación recibida (120.000 €) se destinará al proyecto "Subpoblaciones linfocitarias como biomarcador predictivo de respuesta a inmunoterapia anti-PD1/PDL1 en carcinoma no-microcítico de pulmón avanzado en 1º línea de tratamiento". Este estudio se desarrollará durante los próximos cuatro años y contará con la dirección del Dr. David Escors.
El Museo Reina Sofía acogió ayer la entrega de las ayudas anuales de la AECC, dentro de los actos programados en el Día Mundial de la Investigación en Cáncer (WCRD en sus siglas en inglés), que se comemora cada año el 24 de septiembre. En total, la AECC ha entregado casi 21 millones de euros para financiar 171 proyectos que se suman a los 56M€ con los que hoy se están financiando 380 proyectos de investigación en desarrollo.
Asimismo, la asociación ha puesto de manifiesto la necesidad de elaborar un Plan Nacional de Investigación en Cáncer para alcanzar el 70% de supervivencia media a cinco años en el año 2030, en la actualidad se sitúa en un 53%.
Más información sobre el proyecto
La inmunoterapia antiPD1/PDL1 ha supuesto una revolución en el tratamiento del cáncer no-microcítico de pulmón, ya que ha mejorado los resultados de la quimioterapia, asociando además menor toxicidad. Por desgracia la proporción de pacientes que responden al tratamiento es reducida, son menos todavía los que mantienen la enfermedad controlada durante un periodo de tiempo prolongado, y no se dispone de biomarcadores predictivos que permitan identificar a estos pacientes con precisión.
El equipo del Dr. David Escors ha desarrollado un sistema de monitorización de poblaciones linfocitarias por citometría de flujo a partir de sangre periférica en pacientes en progresión a quimioterapia que permite predecir aquellos que van a responder a la inmunoterapia. Dada la reciente aprobación de la inmunoterapia en pacientes en primera línea, este proyecto pretende correlacionar los perfiles linfocitarios de los pacientes y su dinámica con la eficacia del tratamiento en este contexto, incorporando además el estudio de las citosinas proinmunogénicas en plasma y la posible influencia del daño genotóxico en las células inmunitarias producido por las diferentes terapias como causa de menor eficacia en segunda línea.
El proyecto del Dr. Hugo Arasanz se completará con estudios mecanísticos in vitro que permitan conocer los elementos que condicionan la respuesta al tratamiento y plantear combinaciones que puedan revertir la resistencia primaria a estas terapias.



Navarrabiomed - Centro de investigación biomédica
Complejo Hospitalario de Navarra, edificio de investigación.
Calle Irunlarrea, 3. 31008 Pamplona, Navarra, España.
The team do research mainly on highly sensitive sensor and technical platforms for thin-film and biological substance characterisation using metamaterials, metasurfaces and plasmonic structures. The characterisation is multispectral, that is, it covers the entire infrared spectrum, from the terahertz band or far infrared to the visible infrared, including mid infrared and near infrared.

Navarrabiomed - Centro de investigación biomédica
Complejo Hospitalario de Navarra, edificio de investigación.
Calle Irunlarrea, 3. 31008 Pamplona, Navarra, España.